Россия
BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
This study aimed to investigate the spatial distribution of dissolved iron from river to coastal waters and iron bioavailability for coastal phytoplankton. Dissolved iron concentrations and other water quality parameters (e.g., pH, concentrations of dissolved organic carbon and trace metals, etc.) were determined in the Shizugawa Bay and its adjacent rivers, northeast Japan. Coastal dominant diatom (Chaetoceros sp.) isolated from the bay was used for incubational assay to examine growth kinetics in a range of iron concentrations. As a result, total dissolved iron concentrations of inland waters (75 ± 80 nM) were substantially higher than those of coastal waters (7.2 ± 4.8 nM). Among inland waters, iron concentrations from anthropogenic waters were relatively higher than those for forested river waters. In the bay, relatively higher concentrations of iron were observed in the inner part. From the growth experiment, half-saturation constant of iron for the growth of Chaetoceros sp. was determined to be 1.8 - 3.5 nM. The observed dissolved iron concentrations combined with growth response indicate that growth of Chaetoceros sp. is in some cases limited by iron availability. However, this study generally suggests that, while dissolved iron concentration largely decreased from river to coastal waters, terrestrial iron inputs potentially including both natural and anthropogenic sources contribute sufficient growth and iron availability by Chaetoceros sp. in the Shizugawa Bay.
iron, uptake kinetics, phytoplankton, coastal, terrigenous, anthropogenic
I. INTRODUCTION
Terrestrial nutrients loadings contribute to high productivities in coastal areas providing rich ecosystems and abundant fisheries resources. However, eutrophication by excess nutrient loadings from land to the ocean due to human activities have negative impacts on costal environments, such as occurrences of harmful algal blooms (HABs) and oxygen deficient water. In Japan, HABs by eutrophication due to human activities have caused serious damage to the coastal environments from 1960's [1]. Then, after enforcing regulations for nutrient loads in 1973, eutrophication have been improved resulting om the decrease of red tide events in Japan. On the other hand, low nutrient concentrations have recently been suspected to cause some damage to fisheries, such as the bleaching of cultured seaweeds (Pyropia spp.) in some areas [2, 3]. Thus, in order to preserve the coastal ecosystem with maximum utilization of the coastal environments, such as fisheries and aquaculture, the nutrient dynamics in the coastal regions and the impact of terrestrial loadings on the coastal ecosystems are needed to understand.
Iron (Fe) is an essential micronutrient for marine micro alga [4]. Thus, it limits the algal growth not only in open oceans [5–7] but in coastal areas as well [8] due to the lower solubility of Fe at the circumneutral pH comparing their requirement [9]. Terrestrial iron bound to organic matters, such as humic or fulvic acids is thought to make up most of the bioavailable iron supply to aquatic environments [8, 10, 11]. Although many studies that investigated the dynamics of the river and coastal iron were thought [8, 11–14], the iron dynamics and bioavailabilities from rivers to ocean have hardly been investigated simultaneously. This study aimed to investigate the spatial distribution of dissolved iron from river to coastal waters and iron bioavailability for coastal phytoplankton.
II. MATERIALS AND METHODS
Study site
Shizugawa Bay is a semi enclosed bay with an area of 46.8 km2. It is located in northeastern Japan, facing the Pacific Ocean (Fig. 1). Most river mouths that flow into the bay, including the largest one (Hachiman River), are located in the inner part of the bay. The basins of the flowing river are mostly surrounded by mountains covered with coniferous or deciduous broad-leaved forests (>70%). Oysters and seaweeds are abundantly cultured in the entire area of the bay. The tsunami following the 2011 off the Pacific coast of Tohoku earthquake had severe impacts on the bay and most of its coastal flat land area.
Field samplimg
Seawater samples were collected from 0 and 10 m layers (if water depth was less than 10 m, samples were collected at 8 m) at three fixed stations located in the inner, middle, and outer part of the Shizugawa Bay (Fig. 1) once every three months from July 2014 to April 2015. River water samples were collected from surface at the downstream of the three main rivers (Hacihman, Oritate, and Mizushiri) once every month from July 2014 to April 2015 (Fig. 1). The sample collection was conducted using acid washed plastic bottles, and basic water quality parameters were measured, including water temperature, pH, and electrical conductivity.
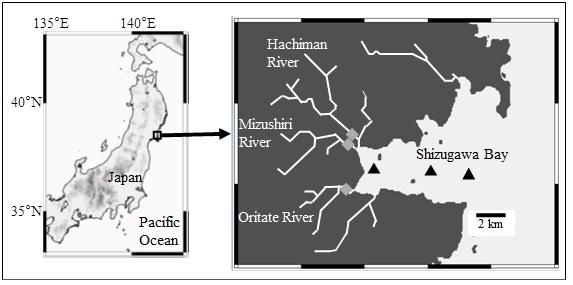
Fig. 1. Sampling location in the Shizugawa Bay, Japan and its main adjacent rivers. ▲and ◆ indicate the stations at the bay and the rivers (Hachiman, Mizushiri, and Oritate Rivers).
Sample procedure
Collected water samples were filtered through a polytetrafluoroethylene membrane filter with 0.45 μm pore size (Merck Millipore). After filtration, water quality parameters, including salinity and concentrations of Fe, dissolved organic carbon, absorbance and fluorescence spectra were measured. The concentrations of dissolved Fe were determined using an inductively coupled plasma mass spectrometer (ICP-MS; 7700x, Agilent Technologies). Before analysis by ICP-MS, excess salt in the seawater samples were removed with solid phase chelate extraction technique using Nobias CHELATE-PA1 (Hitachi High-Technologies Co.; [15]). Because DOM and the specific UV absorbance (SUVA254: ratio of absorbance at a wavelength of 254 nm [A254] relative to the dissolved organic carbon concentration [DOC]) has a significant positive correlation with the metal-to-DOC concentration ratio (a parameter defined by the concentration ratio of dissolved trace metals relative to DOC) for all metals studied consists of a range of organic molecules, of which humic substances (HS) are the primary metal-binding ligands for trace metals including copper and iron.3The concentrations of dissolved organic carbon (DOC) and absorbance at a wavelength of 254 nm [A254] were determined using a TOC analyzer (TOC-5000, Shimadzu) and a UV-visible spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) with 1 cm path-length quartz cuvette. All sample analysis were treated with clean techniques.
Iron uptake experiments
Centric diatom Chaetoceros sp. was isolated from the station at the inner part of the Shizugawa Bay in April 2015 (Fig. 1). From microscopic observation, this species was found to be most dominant in the bay during the sampling period. This clonal strain was rendered axenic by treatment with AM9 antibiotics [16], which was confirmed by epifluorescence microscopy with 4',6-diamidino-2-phenylindole (DAPI) staining method [17].
The strain was maintained with f/2 medium [18], and then 1 mL of the culture was inoculated into 50 mL of 1/10-diluted Aquil* medium [19, 20] with 1/50-diluted Fe (20 nM) and ethylenediaminetetraacetic acid (EDTA, 200 nM) to introduce the Fe limited condition. The first pre-culture was grown for 7 days until it reached the stationary phase, and then 40 mL of this pre-culture was centrifuged at 3000 g for 10 min. The pellet occupied with algal cells were suspended to 40 mL of the artificial 1/10-diluted Aquil* medium without Fe (< 1 nM) and EDTA, and then inoculated for 2 days to prepare the Fe-starved culture of Chaetoceros sp. After the second pre-culture, the iron starved culture was centrifuged at 3,000 g for 10 min, and the pellets were re-suspended in the artificial seawater without any nutrients addition to remove the extracellular Fe. This washing step was conducted twice, and then the algal cells were suspended to the 40 mL of artificial sea water without nutrients. This algal suspension was used for the iron uptake experiments.
As for the culture mediums, 0.4 mL filtrated river water samples were added to 3.5 mL of artificial sea water enriched with 1/10 diluted Aquil* nutrients without Fe and EDTA. Similarly, 3.9 mL of filtrated seawater samples enriched with 1/10 Aquil* nutrients without Fe and EDTA was used for the culture medium. These medium were filter-sterilized with 0.1 µm filters and dispensed into the wells of sterile, plastic, 24-well microplates (IWAKI & Co., LTD.). Finally, 0.1 mL of the iron depleted preculture of Chaetoceros sp. was introduced into the medium with an initial cell density of 240 cells mL-1. These procedures were treated with clean techniques in a clean bench. All medium and nutrient solutions filter-sterilized with 0.1 µm filters. Tests for each medium were triplicated.
These algal cultures were incubated at at 15°C, with a 12-h light:12-h dark cycle and a light intensity of 110 – 130 µmol photons m-2 s-1, and growth was monitored by microscopic cell counts using hemocytometer every other day. Using the cell count data, log phases of at each media were determined, and the specific growth rates (µ [division day-1]) during the log phase were calculated using equation (1) shown below.
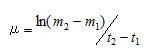 (1)
(1)
Here, t2 and t1 indicate the incubation time (day) of start and end of the log phase, and m2 and m1 indicate the cell density at each incubation time. The specific growth rates were the average value from the triplicated cultures at each sample. The obtained iron concentrations and the specific growth rates of medium using the river and sea water samples were fitted to the following Michaelis–Menten equation.
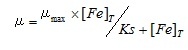 (2)
(2)
Here, µ indicate the specific growth rate at each culture media. In this study, µmax was the maximum growth rate of this alga and was determined to be 1.2, according to the results of the preliminary test using Aquil* medium enriched saturated Fe (1.0 µM) and EDTA (10 µM). [Fe]T indicates the dissolved Fe concentration of each water sample measured with ICP-MS. From these data, half-saturation constants (Ks) was finally calculated. Least-square method was used for fitting the data.
III. RESULTS AND DISCUSSION
Bioavailabilities of Fe
The Ks of iron uptake by the Chaetoceros sp. was calculated as 1.8 and 3.5 nM with river water and seawater samples, respectively (Fig. 2A and 2B). These values were in almost in the same range, suggesting that the terrestrial Fe transported from the rivers to the Shizugawa Bay was bioavailable for the marine phytoplankton. The dFe concentrations of the river water samples were sufficiently higher than those of seawater (Figs. 3 and 4). Thus, transported dFe from rivers to the ocean was considered to contribute to the increase in bioavailable Fe in Shizugawa Bay. On the other hand, dFe concentrations were lower than the Ks values in some cases, especially in the middle areas of the bay through the year, suggesting that iron potentially limits the algal growth in the bay. Thus, distribution of bioavailable Fe was potentially insufficient for algal growth, and Fe concentrations should be considered to
maintain sustainable primary production in the bay.
.jpg)
Dynamics of Iron concentration in river and ocean
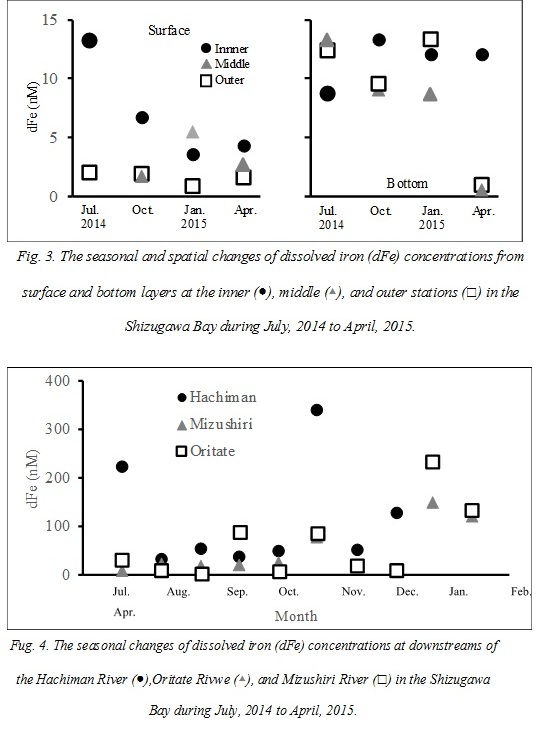
Total dissolved Fe (dFe) concentrations of the coastal waters in Shizugawa Bay ranged from 0.6 to 13.3 nM with an average of 7.2 ± 4.8 nM (Fig. 3). The dFe concentrations at the water surface were significantly lower than those of water at greater depths (9.5 ± 4.3 nM, t-test, p < 0.05), suggesting that iron elution from bottom sediments, Fe decomposition at deeper water, or Fe consumption by phytoplankton at the surface contributes to the iron dynamics in the bay. The dFe concentrations at the surface layer of the inner station in Shizugawa Bay (7.0 ± 3.8 nM) were higher than those at the middle station (1.6 ± 0.4 nM, turkey’s test, p < 0.05), while no significant difference with the outer station (5.8 ± 4.6 nM) was observed. These dFe concentrations in the inner station were higher than those in the surface oceanic waters (0.42 – 3.53 nM from 5 and 10 m depths; [21]) near the Shizugawa Bay, influenced by the Oyashio and Kuroshio currents. These results indicate that terrestrial dFe loading contributes to the increase in the dFe concentrations in the inner part, but the influence of river input was limited to the inner part of the bay. Significant seasonal changes were not observed at neither the surface nor bottom waters in the bay, and the other environmental factors (salinity and Chl a and DOC concentrations) did not show any significant correlation with dFe.
Total dissolved Fe (dFe) concentrations of the river waters varied between 3.9 and 341 nM with an average of 75 ± 80 nM (Fig. 4). These concentrations were relatively lower compared to the previous studies in close-by areas. For example, dFe concentrations of the rivers in the same prefecture of this study were reported to be 0.06 – 2.18 µM in Takagi River [10] and 0.11 – 5.46 µM, the rivers flowing into the Kesennuma Bay [12], significantly lower than those in our study. The populations, agricultural fields, and flow length in the basins of the previous research were much larger and longer, respectively, than the rivers sampled in this study. Moreover, the dFe concentrations of the Hachiman River, where the population is the largest in the basin, were relatively higher than those in the other sampling rivers (Fig. 4). Therefore, the loads or characteristics of a river may potentially affect the Fe dynamics of river in some cases. In fact, the dFe concentrations of some of the anthropogenic waters collected from a septic tank and paddy fields in the Hachiman River basin (160 - 560 nM) were higher than those of the Hachiman river waters.
Some studies suggested that forested river water supplies more bioavailable Fe than urbanized river water [8]. In this study, however, dFe concentrations of the Hachiman River were the highest among the sampling rivers, and it also had the highest population in the basin. Meanwhile, Fe bioavailabilities for coastal diatoms were not different from river to river. Therefore, the present study suggests that both forested and urbanized river waters are both important for coastal productivity. However, the population density in the basin around the sampling site is much lower than the most urbanized regions in Japan such as Tokyo, and thus, Fe loading from urban waters and its bioavailability is still unclear.
The concentrations of dFe in each river (Hachiman, Mizushiri, or Oritate Rivers) significantly correlated with DOC (peason’s simple regression, r = 0.77, 0.70, or 0.69, respectively, p < 0.05; Fig. 5) and [A254] (peason’s simple regression, r = 0.79, 0.65, or 0.73, respectively, p < 0.05; Fig. 5). [A254] of natural water samples is known to show the strong correlation with the amounts of humic substance and both DOC and [A254] are reported to affect the dFe dynamics in the river waters [22–24]. Previous studies revealed that humic like organic matters contributes to the iron solubility in natural rivers as a function of iron chelator [25–27]. Similarly, the present study revealed the positive relationships between dFe concentrations, and DOC and [A254], and thus suggested that humic like substances in the rivers were highly contribute to the transport of bioavailable iron to the sea.
Conclusion
The coastal dFe concentrations were mostly higher than the Ks of iron uptake by the dominant diatom Chaetoceros sp., whereas the dFe concentrations were insufficient in some cases to maintain the Chaetoceros growth, especially in the middle and outer part of the bay, suggesting that Fe potentially limits the algal growth in the Shizugawa Bay. The dFe concentrations in the rivers were much higher than those in the river waters and surface iron concentrations in inner part of the Shizugawa Bay were higher than those in middle and outer part. In addition, the bioavailability of the dFe in the rivers were similar to those in seawater, suggesting the potential contribution of terrestrial iron to the coastal productivity.
IV. ACKNOWLEDEMENT
This study was supported by Grant-in-Aid for Encouragement of Young Scientists (A) 25709045, grant for projects for the production, preservation & restoration of cultural properties outside Japan from the Sumitomo Foundation, and by The Environment Research and Technology Development Fund (S13) from the Japanese ministry of the environment.
1. Imai I, Yamaguchi M, Hori Y (2006) Eutrophication and occurrences of harmful algal blooms in the Seto Inland Sea, Japan. Plankt Benthos Res 1:71-84. doi:https://doi.org/10.3800/pbr.1.71
2. Itakura S, Imai I (2014) Economic impacts of harmful algal blooms on fisheries and aquaculture in western Japan - An overview of interannual variability and interspecies comparison. Proc Work Econ Impacts Harmful Algal Bloom Fish Aquac 17-26.
3. Nishikawa T, Tarutani K, Yamamoto T (2010) Nitrate and phosphate uptake kinetics of the harmful diatom Coscinodiscus wailesii, a causative organism in the bleaching of aquacultured Porphyra thalli. Harmful Algae 9:563-567. doi:https://doi.org/10.1016/j.hal.2010.04.007
4. Sunda WG (2012) Feedback interactions between trace metal nutrients and phytoplankton in the ocean. Front Microbiol 3:1-22. doi:https://doi.org/10.3389/fmicb.2012.00204
5. Hutchins D a., Hare CE, Weaver RS, et al (2002) Phytoplankton iron limitation in the Humboldt Current and Peru Upwelling. Limnol Oceanogr 47:997-1011. doi:https://doi.org/10.4319/lo.2002.47.4.0997
6. Coale KH (2004) Southern Ocean Iron Enrichment Experiment: Carbon Cycling in High- and Low-Si Waters. Science (80- ) 304:408-414. doi:https://doi.org/10.1126/science.1089778
7. Martin JH, Coale KH, Johnson KS, et al (1994) Testing the iron hypothesis in ecosystems of the equatorial Pacific Ocean. Nature 371:123-129.
8. Lewitus AJ, Kawaguchi T, Ditullio GR, Keesee JDM (2004) Iron limitation of phytoplankton in an urbanized vs . forested southeastern U . S . salt marsh estuary. 298:233-254. doi:https://doi.org/10.1016/S0022-0981(03)00361-7
9. Liu X, Millero FJ (1999) The solubility of iron hydroxide in sodium chloride solutions. Geochim Cosmochim Acta 63:3487-3497. doi:https://doi.org/10.1016/S0016-7037(99)00270-7
10. Matsunaga K, Kuma K, Toya K (1998) Riverine input of bioavailable iron supporting phytoplankton growth in Kesennuma Bay. Water Res 32:3436-3442.
11. Nishioka J, Nakatsuka T, Ono K, et al (2014) Quantitative evaluation of iron transport processes in the Sea of Okhotsk. Prog Oceanogr 126:180-193. doi:https://doi.org/10.1016/j.pocean.2014.04.011
12. Fujii M, Sasaki A, Otono S, et al (2006) Spatial and seasonal distributions of dissloved organic matter and iron Matsushima Bay, Japan. Jounal Japan Soc Water Environ 29:169-176.
13. Kuma K, Katsumoto A, Shiga N, et al (2000) Variation of size-fractionated Fe concentrations and Fe(III) hydroxide solubilities during a spring phytoplankton bloom in Funka Bay (Japan). Mar Chem 71:111-123. doi:https://doi.org/10.1016/S0304-4203(00)00044-X
14. Testa JM, Charette MA, Sholkovitz ER, et al (2002) Dissolved iron cycling in the subterranean estuary of a coastal bay: Waquoit Bay, Massachusetts. Biol Bull 203:255-256.
15. Sohrin Y, Urushihara S, Nakatsuka S, et al (2008) Multielemental Determination of GEOTRACES Key Trace Metals in Seawater by ICPMS after Preconcentration Using an Ethylenediaminetriacetic Acid Chelating Resin. Anal Chem 80:6267-6273. doi:https://doi.org/10.1021/ac800500f
16. Provasoli L, Shiraishi K, Lance JR (1959) NUTRITIONAL IDIOSYNCRASIES OF ARTEMIA AND TIGRIOPUS IN MONOXENIC CULTURE*. Ann N Y Acad Sci 77:250-261. doi:https://doi.org/10.1111/j.1749-6632.1959.tb36905.x
17. Porter KG, Feig YS (1980) The use of DAPI for identifying aquatic microfloral. Limnol Oceanogr 25:943-948. doi:https://doi.org/10.4319/lo.1980.25.5.0943
18. Guillard RRL (1975) Culture of Phytoplankton for Feeding Marine Invertebrates BT - Culture of Marine Invertebrate Animals: Proceedings - 1st Conference on Culture of Marine Invertebrate Animals Greenport. In: Smith WL, Chanley MH (eds). Springer US, Boston, MA, pp 29-60
19. Harrison PJ, Berges JA (2005) Marine culture media. Algal Cult Tech 21-34.
20. Morel FMM, Rueter JG, Anderson DM, Guillard RRL (1979) AQUIL: A CHEMICALLY DEFINED PHYTOPLANKTON CULTURE MEDIUM FOR TRACE METAL STUDIES12. J Phycol 15:135-141. doi:https://doi.org/10.1111/j.1529-8817.1979.tb02976.x
21. Takata H, Kuma K, Iwade S, et al (2004) Spatial variability of iron in the surface water of the northwestern North Pacific Ocean. Mar Chem 86:139-157. doi:https://doi.org/10.1016/j.marchem.2003.12.007
22. Maloney KO, Morris DP, Moses CO, Osburn CL (2005) The role of iron and dissolved organic carbon in the absorption of ultraviolet radiation in humic lake water. Biogeochemistry 75:393-407. doi:https://doi.org/10.1007/s10533-005-1675-3
23. Weishaar J, Aiken G, Bergamaschi B, et al (2003) Evaluation of specific ultra-violet absorbance as an indicator of the chemical content of dissolved organic carbon. Environ Sci Technol 37:4702-4708. doi:https://doi.org/10.1021/es030360x
24. Xiao Y-H, Sara-Aho T, Hartikainen H, Vähätalo A V. (2013) Contribution of ferric iron to light absorption by chromophoric dissolved organic matter. Limnol Oceanogr 58:653-662. doi:https://doi.org/10.4319/lo.2013.58.2.0653
25. Laglera LM, van den Berg CMG (2009) Evidence for geochemical control of iron by humic substances in seawater. Limnol Oceanogr 54:610-619. doi:https://doi.org/10.4319/lo.2009.54.2.0610
26. Powell RT, Wilson-Finelli a. (2003) Importance of organic Fe complexing ligands in the Mississippi River plume. Estuar Coast Shelf Sci 58:757-763. doi:https://doi.org/10.1016/S0272-7714(03)00182-3
27. Buck KN, Lohan MC, Berger CJM, Bruland KW (2007) Dissolved iron speciation in two distinct river plumes and an estuary: Implications for riverine iron supply. Limnol Oceanogr 52:843-855. doi:https://doi.org/10.4319/lo.2007.52.2.0843







