BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
Algae has a great potential for quick capture of biological carbon and its storage in saltwater-inundated coastal wetlands and can also be introduced as a climate adaptive alternate farming practice. An intervention with native algal flora Enteromorpha sp. in enclosed coastal Sundarbans in India on two open water culture techniques, viz. U-Lock & Fish-Bone, shows that growth in native algal stock is influenced by seasonal variations of salinity and other limnological factors. Sundarbans, facing the odds of climate change is fast loosing arable lands to sea level rise. Algaculture in inundated coastal areas can be an adaptive mitigation for the same. Perusal of results show that daily growth rate (DGR%) increases with increasing salinity of the intruding tidal waters to an extent and biomass increment under salt stress results in accumulation of metabolites those are having nutrient values and can yield bio-diesel as well. Algal growth recorded mostly in post monsoon period, has impacts on pH and Dissolved Oxygen (DO) of the ambient water to facilitate integrated pisciculture. The paper suggests that alga-culture has unrealized potentials in carbon sequestration and can be significantly used for extraction of Biodiesel.
alga-culture, enclosed coast, sustainable aquafarming, sundarbans, climate change, risk reduction.
I.INTRODUCTION
Deltaic villages in enclosed coasts of Indian Sundarbans, a world heritage site, are extremely vulnerable to rising sea level and sea surface temperature, rendering farmlands of marginal coastal communities non-yielding, owing to increasing soil salinity. The enclosed coastal habitat has natural resources of mangrove forest and enriched biodiversity that extends several ecosystem services for sustaining life and livelihood. It is also one of the most ecologically threatened areas of the planet [5]. Available brackish water environment of Sundarbans provides favourable substratum for algal communities [28]. Enteromorpha intestinalis, Ulvalactuca and Catenellarepens are the dominant macro-algae growing naturally in this habitat and are ecologically important in accommodating other living organisms as reported earlier [23], [24], [24], [29], [30].
These macro-algae have numerous usages, including production of food, feed, fodder, fertilizer and as well,chemical feedstock and bio-fuel [20], [21], [22], [26], [27]. There is mention of their polysaccharides being used in food, cosmetics, paints, crop, textile, paper, rubber and additionally they are used in areas of pharmacology for their antimicrobial, antiviral, antitumor, anticoagulant and fibrinolytic properties. However, culture methods for propagating these local algal flora for commercial usage are recently known [17]and policy instruments are meager in promoting it as a ‘REDD-plus’ implication in coastal areas for conservation of mangrove forests, since it can be used for bio-fixation of CO2 and GHG abatement as it has an estimated potential of sequestering multifold times of CO2 it emits by respiration.
This paper attempts to assess the bio-economic potentials of integrated alga-culture as an alternative livelihood for marginal communities in inundated coastal areas, who have lost farmland to intruding seawater, in assuring sustainable change management towards poverty alleviation in the milieu of climate change.Reports on eco-physiological studies of brackish water algae of Sundarbans and impact of seasonal variation on the biochemical composition of the green weeds have already been reported [19], however, there is substantial knowledge gap in recommending the commercial utilization of these algae in Sundarbans. Similar research on the nutritional evaluation of the seaweeds has been conducted elsewhere and evaluation of varied species of weeds for multiple industries has been reported [38] as well.
Hence the objective of the present study remains toestimatethe seasonal growth of the flora in biomass and simulate the accumulation of the bioactive components in Enteromorpha and Ulva that has nutritional values and as well, has potentials for bio-energy alternatives. Further, the intervention also assesses the sustainability of production in alga culture and as well the carbon mitigation potentials of such aquafarming. Reference of this preliminary work would entail developing and establishing the production processes for a range of products in the food and pharmaceutical sector and as well, a newsource for bio-energy, towards creating carbon smart economic alternativesand opportunities for the relinquished community.
II. METHODOLOGY
Experimental Design & Sample: Algal samples of free floating Enteromorpha intestinalis, Enteromorpha prolifera were collected every month in pre-monsoon (March to June), monsoon (July to October) and post-monsoon (November to February) seasons of the year, from 2012 to 2015, grown from their natural habitat of enclosed coasts of Sagar (21⁰48’N, 88⁰5’59.9”E), Jhorkhali (22⁰13’20”N, 88⁰56”43”E) and Saatjelia (22⁰8’39”N, 88⁰52’40”E)islands in Gosaba block of Indian Sundarbans, and as well from aquaculture ponds, where alga-cum-fish culture is improvised in (a) U-Lock Mass Culture ponds of U-shaped interlocking landscapes in enclosed coasts allowing inundation during high tides and split with fine nylon net partitions for making pen-culture enclosures and (b) Fish-Bone Mass Culture techniques, modified from Organization for Marine Conservation Awareness and Research (OMCAR) model for restoration of mangroves in the coastal habitat, wherein a channel of inflowing tidal water is bifurcated in lateral outlets like a fish-bone structure and partitioned with nylon net. The samples were washed with water and prepared for physical and biochemical studies and as well some sun-dried for a few days, since water inhibits transesterification.
Daily Growth Rate (DGR%): DGR% was calculated using a formula [8]
DGR% = ln (Wf / Wo) / t X 100;
Wherein t is the number of culture days. Wo is the initial fresh weight (g) and Wf is the final fresh weight (g) after culturing for t number of days.
Biochemical Analysis: Limnological parameters of water in the growth ponds were estimated by standard methods following APHA & NEERI (2012). Compositional analysis of algal filaments and frond was done followed by official methods of analysis of AOAC International (19th edition, 2012).Afterwards, dried algae were crushed and extracted in n-hexane in a Soxhlet apparatus as per UNE-EN 734-1 (2006). The transesterification process was conducted simultaneously with the extraction in order to avoid the previous step of oil extraction and purification of obtained oil(Karaosmanoglu et al., 1996; Lang et al., 2001). The protein and carbohydrate content of freeze-dried alga was determined spectrophotometrically[1]. Lipids were extracted from the samples [39] and Fatty Acid Methyle Esters (FAME) &Linolenic acid content was estimated using gas chromatographic (GC) methods conforming to the UNE-EN-12424 (2003) standards, using Bruker 450-GC. GC control and data handling was done using BrukerGalaxie™ Software.
Carbon Mitigation Potential: Algae have the ability to fix carbon dioxide efficiently [35], [2]. The carbon content varies with algal strains, media and cultivation conditions. The CO2 fixation rate can be calculated by applying law of conservation of mass:
Total CO2 fixation = K × biomass productivity X fixation efficiency (wherein K is the rate constant with a value 1.89).
Statistical Analysis: Data were analyzed statistically using ANOVA in <excel>to determine the differences in seasonal growth and composition with different initial seed density along with various seasons of culture period.
III. RESULTS AND DISCUSSION
Green seaweed Enteromorpha intestinalis (L. Link) have high rate of carbon sequestration potentials. It can also withstand the salinity up to 136 ppt[33] and it has been used to determine the nitrogen sources to estuaries [6]. This was also used as an indicator of nutrient enrichment in coastal estuaries and lagoons [10]. The effect of different salinity regime and varying salinity in the growth rate of E. intestinalis has been studied [13]. The positive and negative effect of riverine input in the growth rate of E. intestinalis has been published in Hydrobiologia [18]. Herein, the comparative growth rates, components of nutrient values and seasonal changes in carbon mitigation potential are substantiated with data tables and graphs in the following paragraphs of the text.
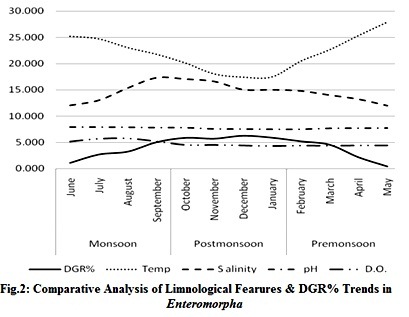
Perusal of result shows that in culture ponds the DGR% varies with the initial inoculation volume and the season of growth as well (Fig 1(a) & 1(b)), whereas, selected limnological parameters of the culture ponds have been seen to vary seasonally and have a correlation with the varying DGR% during pre-monsoon, monsoon and post-monsoon period (Fig.2).
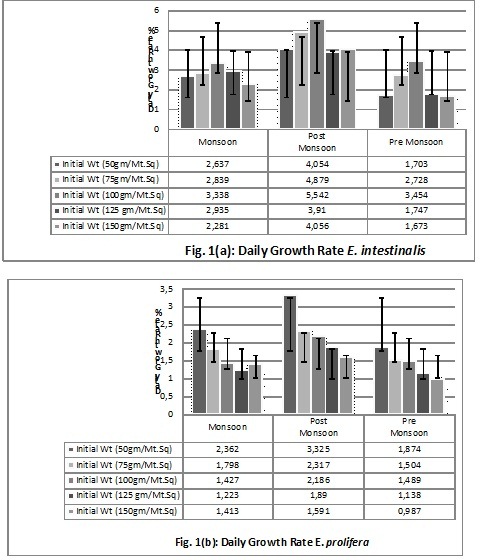
Ambient temperature is maximum during pre-monsoon phase and minimum during winters, showing a negative correlation with salinity. A comparison of variability in DGR% across these seasons and other limnological parameters show direct positive correlation of DGR with salinity. Similar study has reportedly been found in Cyanobacteria that can tolerate higher osmotic stress until salinity reached a PSU of 16 with enhanced growth and synthesis of zeaxanthin[16], [4]. Though, green algae and cyanobacteria from freshwater system shows considerable acclimation towards fluctuating pH, the variability of pH in this case was not as dramatic as salinity. Consistent curves of pH and DO hint a sharp compensation of CO2 release and its utilization buffering the pH with bicarbonates and subsistent release of oxygen through photosynthetic activities [4],[7]. These findings are environmentally relevant to understand the likely impact of salt water intrusion and pH variation on phytoplankton communities in a tropical freshwater system in general and for fish cum algae culture in particular wherein stabilized pH and DO are significant for getting better yield.
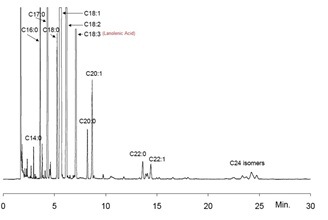
The results showed that in post-monsoon season, available oil in Enteromorpha intestinalis was maximum ranging up to 0.37% which declined to 0.31% during monsoon when average water salinity lowers down, since vegetative growth rate also decreases during monsoon as found in recent studies [17]. However, convertible biodiesel was as high as 93.27% and Linolenic acid content was 0.24% only (Fig. 3) as also reported earlier [14] [15]. Interestingly, the findings of chromatographic analysis also showed that this particular variety of green seaweed is rich in protein, carbohydrate and omega fatty acids which are nutritionally significant. It has been found that the protein content of Enteromorpha intestinalisis as high as 12.9-15.79 %. Also, it reveals that these species contains 53% carbohydrate at an average, which does not show much variation across the seasons, as reported earlier [19], [34]. (Table 1).
|
Table 1: Average Biochemical Composition of Enteromorpha |
|||
|
Biochemical Components |
Pre Monsoon |
Monsoon |
Post Monsoon |
|
Total Lipid (% dry wt) |
12.25±0.57 |
4.58±0.33 |
14.91±1.03 |
|
Protein (% dry wt) |
14.66±1.94 |
12.9±1.58 |
15.79±1.33 |
|
Carbohydrate (% dry wt) |
52.6±2.26 |
53.69±0.91 |
53.66±1.25 |
Thus, it is presumed that utilisation of this species as a part of the human diet after conducting toxicity tests may cater to the acute nutritional deficiency in the region. Its extensive utilisation can parallel and bring a radical change in the lives of Sundarbans inhabitants.
Perusal of Carbon Mitigation Potentials data showed that the maximum optimistic carbonaceous biomass fixation capacity is 2392 Metric Tons per hectare per year, obtainable in U-Lock culture technique during post monsoon season in E.intestinalis, whereas it is lowest in E. prolifera during pre-monsoon season nearing to 442 Ton of algal biomassper hectare per year only. This estimate is drastically higher than carbonaceous biomass fixation capacity of terrestrial plants [32]. It is therefore evident that the accrued biomass through algal growth is a direct evidence of carbon capture by aquatic flora in inundated waters (Fig. 4), as evidenced earlier [3], [7], [12].
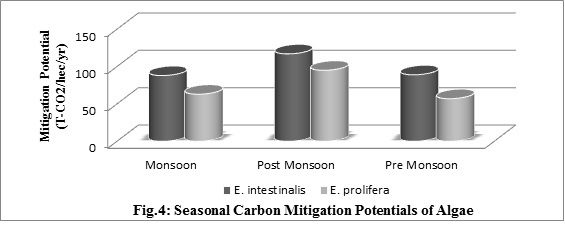
The potential for carbon mitigation in Algal biomass was theoretically assessed [36]. Although, they implemented their assessment for microalgae, we estimated theoretical carbon fixation efficiency of the macroalgae, Enteromorpha intestinalis in three categories: i) optimistic / maximum yield (11.42%), ii) most likely / probable yield (6%) and iii) pessimistic / minimum yield (3%) [37]. Applying the three categories of theoretical efficiency during winter, we observed that the CO2 fixation rate was estimated 14.91, 7.84 and 3.92 g CO2 / m2 / day for maximum, probable and minimum yield respectively. On the other hand, during summer, the rate was 2.88, 1.51 and 0.76 CO2 / m2 / day for maximum, probable and minimum yield respectively.
IV. CONCLUSION
It is obvious that commercial cultivators and end-users for algae shall be interested in the production potentials, availability of food supplements and bio-fuel. The productivity showed sustainable potentials, as may be evidenced in the seasonal rates (Table-2).
|
Table No 2: Comparative Growth Rates & Harvest Potentials of Free-floating Algae |
|||||
|
COMPARATIVE GROWTH RATE & HARVEST POTENTIALS (NATURAL POND WATER) |
|||||
|
Inoculation: Initial Wt=100gm & 50gm/Mt. Sq respectively; SALINITY 2-3 psu |
|||||
|
CULTIVATION MODEL TYPE |
E. intestinalis |
E. prolifera |
|||
|
Av. DGR (%) |
Yield per Week (MT/Hec) |
Av. DGR (%) |
Yield per Week (MT/Hec) |
||
|
Monsoon |
U-LOCK |
3.805 |
7.63 |
2.789 |
6.67 |
|
FISH-BONE |
1.982 |
6.55 |
1.65 |
5.93 |
|
|
FREE-FLOATING |
2.806 |
7.29 |
1.645 |
6.06 |
|
|
Post Monsoon |
U-LOCK |
5.773 |
9.72 |
4.293 |
8.54 |
|
FISH-BONE |
3.775 |
9.03 |
2.551 |
7.91 |
|
|
FREE-FLOATING |
4.488 |
7.45 |
2.262 |
6.5 |
|
|
Pre Monsoon |
U-LOCK |
3.972 |
6.26 |
2.011 |
7.25 |
|
FISH-BONE |
3.481 |
6.59 |
1.832 |
6.47 |
|
|
FREE-FLOATING |
2.261 |
8.81 |
1.398 |
5.73 |
|
|
COMPARATIVE GROWTH RATE & HARVEST POTENTIALS (TRAPPED COASTAL WATERS) |
|||||
|
Inoculation: Initial Wt=100gm & 50gm/Mt. Sq respectively; SALINITY 17-18 psu |
|||||
|
CULTIVATION MODEL TYPE |
E. intestinalis |
E. prolifera |
|||
|
Av. DGR (%) |
Yield per Week (MT/Hec) |
Av. DGR (%) |
Yield per Week (MT/Hec) |
||
|
Monsoon |
U-LOCK |
9.88 |
25.94 |
8.05 |
16.68 |
|
FISH-BONE |
8.36 |
23.58 |
9.5 |
23.06 |
|
|
FREE-FLOATING |
3.45 |
9.45 |
2.98 |
8.33 |
|
|
Post Monsoon |
U-LOCK |
18.72 |
45.79 |
17.23 |
43.65 |
|
FISH-BONE |
16.71 |
41.22 |
17.56 |
43.12 |
|
|
FREE-FLOATING |
7.79 |
12.34 |
3.36 |
10.24 |
|
|
Pre Monsoon |
U-LOCK |
8.39 |
18.91 |
10.07 |
15.15 |
|
FISH-BONE |
7.62 |
17.25 |
7.22 |
16.73 |
|
|
FREE-FLOATING |
3.28 |
9.16 |
3.06 |
10.08 |
|
So, the greenhouse gas benefits from algae culture arise only as offsets when the algal use displaces the combustion of a fossil fuel or used for the production of electricity. Earlier reports from FAO 2010 (http://www.fao.org/uploads/media/0903_CSIRO_Greenhouse_gas_sequestration_by_algae.pdf) showed that it is possible to produce algal biodiesel at less cost and with a substantial greenhouse gas and energy balance advantage over fossil diesel. However, the economic viability is highly dependent upon algae with high oil yields capable of high production year-round, which has yet to be demonstrated on a commercial scale.
This paper substantiates the bio-economic potentials of aqua-farming of the algae Enteromorpha intestinalis as a place based climate adaptive intervention in climate vulnerable deltaic Sundarbans of India, since mangrove ecosystems in Indian Sundarbans are known sources for methane, having very high global warming potential [11]. While higher optimistic carbonaceous biomass fixation capacity of Enteromorpha can be considered as a direct indication of carbon capture by this aquatic flora in inundated waters, as evidenced earlier [17],[12], perusal of results from the present study shows its estimable potential for being used as a source of both bio-diesel and food supplement.
The relevance of this study finds its significance in the fact that the settlement areas of deltaic Sundarbans has unusually high emission footprints in power and transport sectors as reported by WWF (2012), since these desolate islands are not yet connected to the country’s national power grid, whereas loss of agricultural land due to rapid coastal erosion and inundation [31] has accentuated the need for alternatives in food, feed and fodder. This research has all impending merits to find a local need-based solution for combating impacts of global changes.
V. ACKNOWLEDGEMENT
We sincerely acknowledge the support from Asia Pacific Network for Global Change Research (APN-GCR) Japan and Ministry of Environment, Forest & Climate Change, Govt. of India for sponsoring this study to SAFE. We also acknowledge the support and resource sharing of our research collaborator Korea Green Foundation, Central Inland Fisheries Research Institute (CIFRI) of Indian Council of Agricultural research (ICAR) and the Indian Institute of Technology, IIT Kharagpur, and West Bengal.
1. Bannerjee, K. R. Ghosh, S. Homechoudhury&Mitra A (2009). Biochemical Composition of Marine Macroalgae from Gangetic Delta at the Apex of Bay of Bengal. African Journal of Basic & Applied Sciences (ISSN 2079-2034), 1 (5-6), 96-104.
2. Benemann, J., 1997. CO2 mitigation with microalgae systems. Energ. Convers. Manag., 38: 475-479.
3. Campbell P K, T Beer, D Batten (2012) Greenhouse Gas Sequestration By Algae -Energy And Greenhouse Gas Life Cycle Studies. Transport Biofuels Stream, CSIRO Energy Transformed Flagship PB1, Aspendale, Vic. 3195, Australia
4. Chakraborty P, T Acharyya, P V R Babu and D Bandhyopadhyay (2011) Impact of salinity and pH on phytoplankton community in a tropical freshwater system: An investigation with pigment analysis by HPLC. Journal of Environmental Monitoring vol.13(3): 614-620
5. Chaudhuri, A.B. & Choudhury, A. (1994). Mangroves of the Sundarbans. 1, 178-183.
6. Cohen RA and Fong P (2005) Experimental evidence supports the use of δ15 N content of the opportunistic green macro alga Enteromorphaintestinalis (chlorophyta) to determine nitrogen sources to estuaries; Journal of Phycology 41: 287-293.
7. Chi Z, O'Fallon JV, Chen S Trends Biotechnol (2011) Bicarbonate produced from carbon capture for algae culture. 29(11):537-41. doi:https://doi.org/10.1016/j.tibtech.2011.06.006
8. Dawes C J, C Kovach and M. Friedlander (1993) Exposure of Gracilaria to various environmental conditions II. The effect on fatty acid composition. Botanica Marina 36: 289-206
9. European Standard EN 14214 (2003 & 2006). Automative fuels Fatty Acid Methyl Esters (FAME) for diesel engines. Requirements and test methods. CEN - European committee for standarization, Brussels, Belgium.
10. Fong P, Boyer KE and Jedler JB (1998) Developing an indicator of nutrient enrichment in coastal estuaries and lagoons using tissue nitrogen content of the opportunistic alga, Enteromorphaintestinalis (L. Link); Journal of Experimental Marine Biology and Ecology 8(3): 251-258.
11. Jha, C.S., Rodda, S.R, Thumaty, K.C., Raha, A.K., &Dadhwa V.K. (2014). Eddy covariance based methane flux in Sundarbans mangroves, India J. Earth Syst. Sci. 123(5), 1089-1096 Retrieved fromhttp://www.ias.ac.in/jess/jul2014/1089.pdf
12. Kaladharan P, S Veena and E Vivekanandan (2011) Working Paper, Visakhapatnam Regional Centre of Central Marine Fisheries Research Institute, Visakhapatnam 530 003, India
13. Kamar K and Fong P (2000) A fluctuating salinity regime mitigates the negative effects of reduced salinity on the estuarine macroalga, Enteromorphaintestinalis (L.) link; Journal of Experimental Marine Biology and Ecology 254: 53-69.
14. Karaosmanoglu, F. Cigizoglu, K.B. Tuter, M. & Ertekin, S. (1996). Investigation of the refining step of biodiesel production, Energy Fuels 10, 890-895.
15. Lang, X. Dalai, A.K. Bakhshi, N.N. Reaney, M.J. & Hertz, P.B. (2001) Preparation and characterisation of biodiesels from various bio oils, Bioresoure Technology. 80, 53-62.
16. Liua W, Doris W T , Donald M. Andersonb, Paul K S Lama & Rudolf S SWua (2010) Effects of nutrients, salinity, pH and light: dark cycle on the production of reactive oxygen species in the alga Chattonella marina. Working paper No 162, Centre for Coastal Pollution and Conservation, City University of Hong Kong, Kowloon, Hong Kong SAR, China
17. Maity, A. & Dey, D. (2014) Promoting Algaculture in Trapped Waters as Sustainable Aquafarming and Adaptive Climate Mitigation in Inundated Coastal Areas. APN Science Bulletin (ISSN 2185-761x), 4, 170-173.
18. McAvoy KM and Klug JL (2005) Positive and negative effects of riverine input on the estuarine green alga Ulvaintestinalis (syn. Enteromorphaintestinalis) (Linneaus); Hydrobiologia 545:1-9.
19. MitraAbhijit, BanerjeeKakoli, GhoshRajrupaand HomechaudhuriSumit, 2009.Biochemical Composition of Marine Macroalgae from Gangetic Delta at the Apex of Bay of Bengal, African Journal of Basic & Applied Sciences 1 (5-6): 96-104, 2009 ISSN 2079-2034
20. Naskar and A. Bhattacharya, Central Inland Fisheries Institute (ICAR), Kolkata, India. pp:49-66.
21. Naskar NM and Naskar KR. 2007. Diversity of Ulotrichales in estuarine wetlands of North 24-Parganas district, West Bengal.Indian Hydrobiology 10(2): 249-255.
22. Naskar NM, Naskar KR and Sen CR. 2007.Systematic Account and Ecology of Cholorococcales from BrackishwaterBheries (Wetlands) of North 24-Parganas District of West Bengal. Geobios 34:17-20.
23. Naskar NM, Naskar KR and Sen CR. 2008a.Brackishwater Oscillatoriaceae from North 24 Parganas district, West Bengal.Bangladesh J. Plant Taxon 15(1): 31-38.
24. Naskar NM, Naskar KR and Sen CR. 2008c.A new record of blue-green alga LyngbyaspirulinoidesGomont - (cyanobacterium) from West Bengal, India. Seshaiyana 16(3): 3-4.
25. Naskar NM, Naskar KR and Talai S. 2008b. Occurrence of six species of SpirulinaTurpin Em Gardner from the coastal wetlands of North 24 Parganasdistrict,West Bengal, India. Seshaiyana 15(1): 4-5.
26. Naskar NM, Naskar KR and Talai S. 2009.Addition to the list of brackishwaterZygnemaceae of Sundarbans and its adjoining areas, India. Genus Spirogyra Link. Our Nature 7: 187-192.
27. Naskar NM. 2007. First report of DermocarpasphericaSetchelletGardner from West Bengal. Environ and Ecol. 25(3): 719-720.
28. Naskar, N. and Naskar, K.R. 2010. BrackishWaterAlage of Sundarbans, India:Sundarbans, Brackish water, Wetlands, Algal diversity, Ecosystems. Lambert Academic Publishing (LAP), Germany.
29. Naskar, N.M. 2011a. Cyanobacterial biodiversity from brackish water wetlands of Sundarbans. In: Sundarbans:Issues and threats, Eds. K.R.
30. Naskar, N.M. 2011b. Ecosystem functions of Coastal fisheries from Sundarbans: Do algal species matter? In: Emerging trends in Plant Science. Eds. P.K. Das, A.K. Das and N. Das Barasat Govt. College, India.pp: 51-59.
31. Rahman, M. (2012). Time-Series Analysis Of Coastal Erosion In The Sundarbans Mangrove M. International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, Volume XXXIX-B8, and XXII ISPRS Congress, 25 August - 01 September 2012, Melbourne, Australia
32. Ravindranath, N.H. and P.R. Bhat, 1997. Monitoring of carbon abatement in forestry projects-case study of western ghat project, Mitigation and adaptation strategies for Global change, 2: 217-230.
33. Reed and Russel (1979) Adaptation to salinity stress in populations of Enteromorphaintestinalis (L.) Link; Estuarine and Coastal Marine Science Volume 8, Issue 3, March 1979, Pages 251-258.
34. Reeta, J., M.L.Ramalingam, N. Kaliaperumal (2009) Biochemical Composition of Some Green Algae from Mandapam Coast, Res. Bulletin: Regional Centre of Central Marine Fisheries Research Institute, Mandapam, India. 45-56
35. Richmond, A., 2000. Microalgal biotechnology at the turn of the millennium: a personal view. J. Appl. Phycol., 12: 441-451.
36. Sudhakar, K & M. Premlatha (2012) Theoretical Assessment of Algal Biomass Potential for Carbon Mitigation and Biofuel Production. Iranica Journal of Energy & Environment 3 (3): 232-240, 2012
37. Williams, P.J.L. and L.M.L. Laurens, 2010. Microalgae as biodiesel and biomass feedstocks: review and analysis of the biochemistry, energetics and economics, Energy Environ, Sci., 3: 554-590.
38. Wong, K.H. & P.C.K. Cheung, (2001). Nutritional evaluation of some subtropical red and green seaweeds part II In-vitro protein digestibility and amino acid profiles of protein concentrate. Food Chem., 72: 11-7.
39. Yen, R., Zhang, X. &YuzhuLi (2010). African Journal of Biotechnology. 9(33), 5465-5464.







