BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
Blue Carbon, which is carbon captured by marine organisms, has recently come into focus as an important factor for climate change initiatives. This carbon is stored in vegetated coastal ecosystems, specifically mangrove forests, seagrass beds and salt marshes. The recognition of the C sequestration value of vegetated coastal ecosystems provides a strong argument for their protection and restoration. Therefore, it is necessary to improve scientific understanding of the mechanisms that stock control C in these ecosystems. However, the contribution of Blue Carbon sequestration to atmospheric CO2 in shallow waters is as yet unclear, since investigations and analysis technology are ongoing. In this study, Blue Carbon sinks by Zostera marina were evaluated in artificial (Gotenba) and natural (Matsunase) Zostera beds in Ise Bay, Japan. 12-hour continuous in situ photosynthesis and oxygen consumption measurements were performed in both areas by using chambers in light and dark conditions. The production and dead amount of Zostera marina shoots were estimated by standing stock measurements every month. It is estimated that the amount of carbon storage as Blue Carbon was 237g-C/m2/year and 197g-C/m2/year in the artificial and natural Zostera marina beds, respectively. These results indicated that Zostera marina plays a role towards sinking Blue Carbon.
Blue Carbon, Zostera Marina, Photosynthesis, respiration
I. Introduction
In 2009, UNEP proposed that Blue Carbon is carbon that is captured by marine organisms [1]. The amount of total captured carbon in global mean photosynthesis was estimated at about 55%. In Japan, the coastline runs for 35,000km, and is sixth in the world when compared to total land area [2]. Therefore, if Japan is seen as a major carbon stock nation, it receives much benefit from coastal ecosystems. The recognition of the C sequestration value of vegetated coastal ecosystems provides a strong argument for their protection and restoration. Therefore, it is necessary to improve scientific understanding of the mechanisms that stock control C in these ecosystems. However, the contribution of Blue Carbon sequestration to atmospheric CO2 in shallow waters is as yet unclear, since investigations and analysis technology are ongoing. In this study, Blue Carbon sinks by Zostera marina were evaluated monetarily in artificial (Gotenba) and natural (Matsunase) Zostera beds in Ise Bay, Japan.
II. Experimental
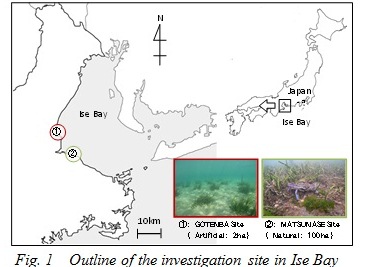
Outline of the investigation site
Investigated Zostera beds in Ise Bay are shown in Fig. 1. The one in Gotenba is an artificial Zostera bed, which was vegetated by the Mie prefectural government in 2008. The initial vegetated area was approximately 700m2, spreading to about 20,000m2 in 6 years. The Zostera beds in Matsunase are the largest natural beds in Ise Bay, and grow along the coastline for about 1,000,000m2. These 2 types of Zostera beds were investigated in this study.
Survey of standing stock of Zostera marina
Zostera marina population and population density were measured with a stratified clipping method at both sites (Fig. 1). After sampling, Zostera marina shoots were separated into roots, seeds, reproductive shoots and vegetative shoots. Then dry weights were measured after drying them at 60℃ for 48 hours. The Carbon content in these parts of Zostera marina were measured by CN coder (Vario Max, Elementar Co. Ltd.), after removing inorganic carbon by acid treatment. These samplings were conducted every 2 months from July 2014 to May 2015.
Survey of photosynthesis and respiration rate of Zostera marina
The photosynthesis and respiration rates in the Gotenba Zostera beds were estimated using light and dark in situ benthic chamber methods. Both types of chambers were placed on the bare sea floor and Zostera beds, and the change in the dissolved oxygen concentration in the chambers was measured for 90 minutes by stirring. The Zostera benthic chamber was always set on three zostera shoots. Before measuring, macro fauna and attached algae were removed by scuba divers. The primary production in sea water was measured at the same time, using the dark and light bottle method. The photosynthesis rates of Zostera marina were estimated by using the equations below:
C=(DODC – DODB – DODW) ・V/S/t eq. (1)
P={(DOLC-DOLB – DOLW) - (DODC-DODB – DODW)}・V/S/t eq. (2)
C indicates the respiration rate, and P indicates the gross primary production rates of Zostera marina. DOLC indicates the amount of change in dissolved oxygen in the light Zostera chamber and DOLB the change in the light benthic chamber. DODC indicates the amount of change in dissolved oxygen in the dark Zostera chamber, while DODB indicates the change in the dark benthic chamber. DOLW and DODW indicate the amount of change in dissolved oxygen in the light and dark sea water bottles, respectively. V indicates the volume of the chamber. S indicates the area of the base of the chamber. T indicates the measuring time. The amount of carbon fixation and emission were estimated by utilizing the photosynthesis rate and respiration rates, assuming that the respiratory quotient remains at 1.0. These investigations were conducted every 2 months from July 2014 to May 2015.
Estimation of the carbon budget of Zostera marina
Zostera marina fix the carbon from sea water by photosynthesis, while at the same time releasing the carbon into the seawater through respiration and decomposition. The carbon budgets were estimated by totalling production, decomposition and respiration (Fig. 2).
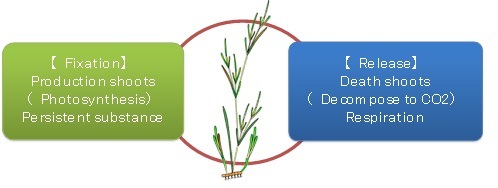
Fig. 2 Carbon budget of Zostera marina
III. Results and discussion
Carbon contents of Zostera marina shoots
Carbon contents in each section of the Zostera marina shoots are shown in Fig. 3. The vegetative shoots and roots could be harvested throughout the year, but the reproductive shoots and seeds could only be harvested from May to September. The carbon contents of the reproductive and the vegetative shoots were 33.2% and 33.5%, respectively. That of the roots were 41.2%, and seeds were 51.3%. It is assumed that the roots and seeds contain a higher level of starch than the vegetative and reproductive shoots [3].
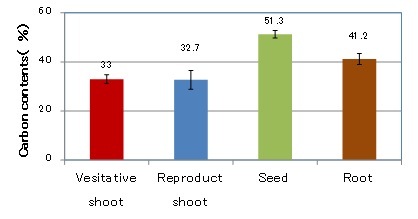
Fig. 3 Carbon contents in each section of Zostera marina
Estimation of amount of shoot production and death shoots
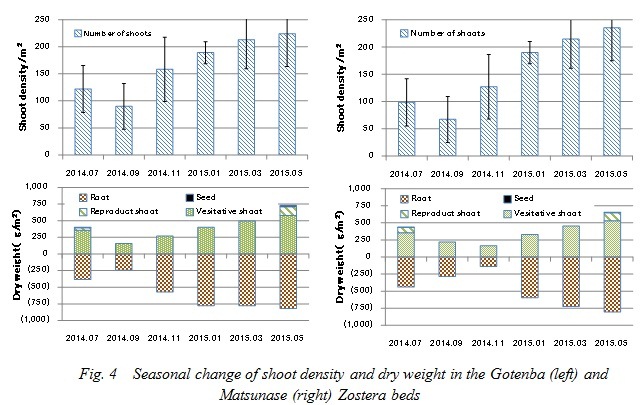
The shoot density and dry weight in each section of Zostera marina at the Gotenba and Matsunase sites are shown in Fig. 4. In both sites, the shoot density and dry weight reached a maximum in May. The dry weight of the aerial part and underground part at the Gotenba site were 762g-DW and 998g-DW, respectively. Those at the Matsunase site were 742g-DW and 992g-DW. The minimum value of shoot density in both sites can be seen in September. The minimum value of dry weight at the Gotenba site was also in September, with the value of the aerial part and underground part being 262g-DW and 358g-DW, respectively. In Matsunase the minimum value of the aerial part and underground part were 202g-DW and 240g-DW, as seen in November.
The above results indicate that the amount of Zostera marina at both sites came in huge waves over a seasonal cycle, and the growth situation was not much difference between the artificial and natural Zostera beds. The amount of shoot production and death shoots were estimated using the following equation:

P indicates the shoot production of Zostera marina. M indicates the death shoots. Cs, Cm, Ce, Cr indicate the carbon content per unit area of the seeds, reproductive and vegetative shoots, and the roots, respectively, while Ws, Wm, We, Wr indicate their respective dry weight per unit area. T indicates the observation periods. The shoot production at the Gotenba and Matsunase sites were estimated at 361g-C/m2/year and 419g-C/m2/year, respectively, and the death shoots were estimated at 122g-C/m2/year and 168g-C/m2/year (Table 1).
Estimation of amount of respiration and photosynthesis
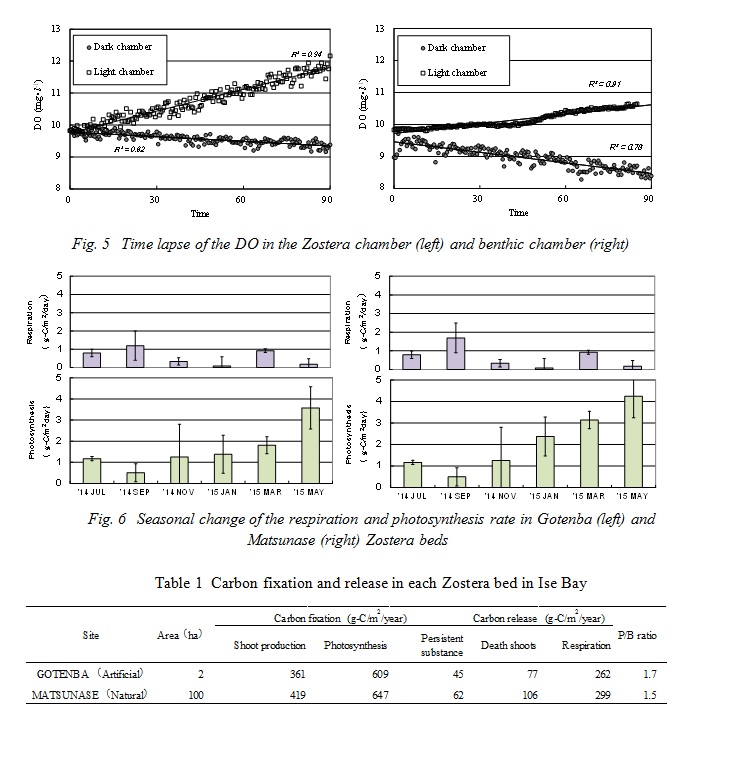
A time lapse of the dissolved oxygen in the Zostera chamber and benthic chamber under light and dark conditions are shown in Fig. 5. The dissolved oxygen in light condition chambers increased in a linear fashion. It is assumed that the Zostera shoots and benthic algae carried on photosynthesis, and that in the Zostera beds especially, supersaturated oxygen was released by photosynthesis. On the other hand, the dissolved oxygen in the dark condition chambers decreased in a linear fashion. This shows that the dissolved oxygen in dark conditions decreased due to the respiration of the Zostera shoots, benthic organisms and bacteria. Next, the amount of photosynthesis and respiration rates by Zostera shoots were estimated using equations (1) and (2). The seasonal change of these rates, after they were corrected for shoot density, is shown in Fig. 6. The photosynthesis rate in Gotenba seasonally changed the same as the standing stock. The maximum value of the photosynthesis rate was 3.57g-C/m2/day in May. The minimum value of the photosynthesis rate was 0.50g-C/m2/day in September. The maximum value of the respiration rate in Gotenba was 1.12g-C/m2/day in September, when the water temperature reached its maximum. The minimum value of the respiration rate was 0.08g-C/m2/day in Sept., when the water temperature reached its minimum. From year-round investigations, the annual carbon release by respiration and carbon fixation by photosynthesis is shown in Table 1. The amount of respiration at both sites was 262g-C/m2/year and 299g-C/m2/year, respectively, and photosynthesis was 609g-C/m2/year and 647g-C/m2/year. The annual production biomass ratios (P/B ratio) of Zostera marina at both sites were calculated from annual carbon fixation by photosynthesis and the maximum standing stock of Zostera shoots. These values were 1.7 in Gotenba and 1.5 in Matsunase. These were lower than Zostera beds in Sanriku and the Seto coast [4].
Carbon budge in Zostera beds
From the above results, the amounts of carbon fixation and release in Zostera beds are shown in Table 1. Carbon fixation was calculated using photosynthesis ratio, and carbon release was calculated from the sum of the death shoots and respiration. These values at both sites were 384g-C/m2/year and 467g-C/m2/year, respectively. The amount of respiration was about 2 times larger than that of death shoots.
The carbon budgets calculated from the sum of carbon fixation and release at the Gotenba and Matsunase sites were estimated at 225g-C/m2/year and 180g-C/m2/year, respectively for fixation. This value at the artificial Gotenba site was higher than that of the natural Matsunase site. Matsunase has the largest natural Zostera marina beds in Ise Bay, with an area of more than 100ha, and they have had a stable existence there for a long time. The Zostera marina beds in Gotenba, however, were artificially planted over approximately 0.1ha. 10 years later, these areas had expanded to about 2ha and are still expanding. That is one of the possible reasons why the production at Gotenba was higher than at Matsunase.
Ito et. al. estimated the annual carbon fixations of typical seaweed and seagrass such as Zostera marina, Eisenia bicyclis, Saccharina japonica and Sargassum beds in Japan at values of 100g-C/m2/year, 1670g-C/m2/year, 130g-C/m2/year, and 600g-C/m2/year, respectively [6]. Otani et. al. estimated a net production of the temperate forest at 650g-C/m2/year [7]. The carbon fixation of Zostera marina in Ise Bay was about a third that of the temperate forest and a sixth that of the Sargassum beds.
However, in this study, a dynamic analysis of death shoots was not conducted. Ito et. al. reported that about 16% of death shoots accumulated with the sediment and had existed for a long period [5]. In this study, it is incorrectly assumed that all death shoots decomposed into carbon dioxide. But if the amount of persistent substance in death shoots is evaluated, there is a potential for an increase in carbon fixation. Therefore, it is thought that decomposition processes of death shoots must be followed-up in detail.
Evaluation of the monetary value of carbon fixation in Zostera beds
The benefits of tidal flats and seagrass beds has been difficult to visualize, especially the direct monetary value of carbon fixation with regards to the transfer of rights to emit carbon dioxide. In this study, the monetary value of carbon fixation in Zostera beds was estimated using an anamnestic knowledge. From the foregoing results, the carbon fixations at the Gotenba and Matsunase sites could be estimated at 225g-C/m2/year and 180g-C/m2/year, respectively. The monetary value of carbon fixation was calculated using a transaction price of carbon dioxide emission rights, set to 610 Japanese yen/t-CO2 by Japan's Voluntary Emissions Trading Scheme in 2010 [8]. Therefore, in the case of 1ha of constructed Zostera bed, it is expected that about 4,500 yen/ha/year was created as a carbon dioxide emission value. The legal duration of Zostera beds is specified as 10 years by ministerial ordinance [9], making the monetary value of carbon fixation about 45,000 yen/ha.
IV. Conclusion
In this study, Blue Carbon sinks and a monetary value for carbon fixation by Zostera marina were evaluated in artificial and natural Zostera beds in Ise Bay, Japan.
The carbon contents of the reproductive and vegetative shoots, roots and seeds were 33.2%, 33.5%, 41.2% and 51.3%. Shoot production at the Gotenba and Matsunase sites could be estimated at 361g-C/m2/year and 419g-C/m2/year, respectively, with the death shoots at both sites at 122g-C/m2/year and 168g-C/m2/year.
The amount of respiration could be estimated at 262g-C/m2/year and 299g-C/m2/year, respectively, and those of photosynthesis at 609g-C/m2/year and 647g-C/m2/year.
The carbon fixations at the Gotenba and Matsunase sites could be estimated at 225g-C/m2/year and 180g-C/m2/year, respectively. In conclusion, the present study has suggested that the Zostera beds in Gotenba and Matsunase were fixed at 4.5t-C/year and 180t-C/year as Blue Carbon, with a benefit of about 45,000yen/ha.
V. Acknowledgments
This study was conducted at the Environment Research and Technology Development Fund. I am deeply grateful to the Mie Prefecture Fisheries Infrastructure Division, who provided carefully considered feedback and valuable comments. Special thanks also to the Fisheries Cooperative Association of Matsusaka for their technical help and sincere encouragement.
1. Nellemann, C.; Corcoran, E.; Duarte, C.M.; Valdes, L.; DeYoung, C.; Fonseca, L.; Grimsditch, G. (Ed.) Blue carbon: The role of healthy oceans in binding carbon. A Rapid Response Assessment. United Nations Environment Programme, GRIDArendal, 2009, pp.1-78.
2. Chronological Scientific Tables of 2010, National Astronomical Observatory of Japan, Maruzen Co. Ltd, 2009, pp.1041.
3. Arasaki S., Study on the Ecology of Zostera marina and Zostera japonica,Japanese Society of Fisheries Science, Vol.16, No. 2, 1950, pp.70-76.
4. National Research Institute of Fisheries and Environment of Inland Sea, Technological Development Projects for Global Warming Countermeasures scheme “Evaluation of the Carbon Fixation Resource in Tidal Flat and Sea Weed Beds and Development of Enhancing Function of the Carbon Fixation, 2014, pp71-90.
5. Carlos M. Duarte and Just Cebrian, The fate of marine autotrophic production, Li, nol. Oceanogr., 41(8), 1996, pp. 1758-1766.
6. Yasushi Ito, Yoshio Nakano, Satoshi Murashita, Nobuo Mikami, Jun Yokoyama, Shinji Kirihara, Masahiro Noto, Estimations of Quantities of Carbon Storage by Seaweed and Seagrass Beds, Japanese Society of Fisheries Engineering, 46, 2009, pp.135-146.
7. Yoshikazu Otani, Carbon Dioxide Fluxes, Japanese Forestry Society, 33, 2001, pp.10-17.
8. Press release information, 2014, Ministry of the Environment, http://www.env.go.jp/press/16213.html.
9. Ministerial ordinance of depreciable asset life, Ministry of Finance, Japan, 2014.







