BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
The impacts of human-induced changes in coastal environments on shellfish farming need to be mitigated. Suspended farming species, such as oysters, greatly impact planktonic communities and benthic environments via filter feeding and bio-deposition. To more effec-tively manage coastal environments and achieve ecologically sustainable shellfish farming, interactions between coastal marine environments and aquaculture activities need to be properly assessed. We examined interactions between coastal biogeochemical environments and suspended oyster farming in Shizugawa Bay of northeastern Japan. We found that particulate organic matter (POM) produced at the oyster farm (e.g., exfoliated periphyton and/or oyster feces) locally increased the concentrations of chlorophyll a and daytime dis-solved oxygen in the bottom layer. Based on the estimated budget of POM at the bay scale, the oyster feeding rate was a couple of orders of magnitude lower than the net primary production and POM inputs at the bay boundaries (e.g., offshore and in rivers). The rela-tively high exposure of the bay and high seawater mixing rate may explain the lack of mac-roscale environmental impacts of oyster cultures at the bay scale. We also found that despite the oligotrophic environment, the oyster growth rate was higher in the bay, compared with previous estimates in other coastal areas. To understand the mechanisms sustaining the production of phytoplankton and oysters, further examinations from the perspective of nu-trient cycling in the bay are required.
oyster, plankton, sediment, chlorophyll a, particulate organic carbon flux, Shizugawa Bay
I. INTRODUCTION
Aquaculture has developed rapidly worldwide over the past 50 years in order to meet the increasing global demand for aquatic products owing to human population growth [1]. In Shizugawa Bay, which is located in the Tohoku Region on the northeast side of the main Japanese island, Honshu, people have made enormous efforts to recover the aquaculture industry from damage sustained by the devastating March 2011 earthquake and tsunami. Before the tsunami, aquaculture facilities were distributed throughout the bay at a high density, but they had various negative impacts such as deterioration of the benthic environment and slow growth of cultivated oysters as in [2] and personal communications with local fishermen. However, for aquaculture recovery in the bay after the tsunami, local fishery committees and fishermen have made great efforts to develop a new sustainable design. In particular, they have reduced the density of aquaculture facilities to mitigate their environmental impacts and enhance oyster production, which has recently led to the Aquaculture Stewardship Council standard certification by the World Wildlife Foundation in 2016.
In general, shellfish aquaculture does not require artificial food supplements for cultured organisms and accordingly is generally considered environmentally friendly and sustainable. In addition, phytoplankton is consumed by shellfish via filter feeding, and substantial nutrients are removed from marine systems during harvests [3]. These processes are thought to mitigate eutrophication in coastal marine systems. However, the opposite effect also has been recognized; shellfish farms increase organic matter and nutrient transport to sediment via the deposition of feces and pseudo-feces. For instance, at a mussel farm in the Northwestern Adriatic Sea (Italy), the depositional fluxes of nitrogen and phosphorus are almost two and five times higher due to oyster feces compared with those in the areas after harvest [4]. Furthermore, introducing high-density shellfish to aquaculture farms alters planktonic community compositions and biomass [1].
The effects of mussels (e.g., Mytilus edulis) and oysters (e.g., Crassostrea gigas), which are important aquacultured shellfish in many regions of the world, on planktonic communities have been extensively investigated at various scales. For instance, a microcosm experiment [5], in-situ mesocosm experiments [6, 7], and field investigations [8-10] in different regions have demonstrated that mussels and oysters significantly reduce phytoplankton biomass. However, opposite results have also been reported; that is, mussel farming did not significantly reduce phytoplankton concentrations [11, 14]. For those cases, mussels may have increased nutrient regeneration and enhanced phytoplankton production. Overall, shellfish aquaculture likely shows either positive or negative effects on surrounding ecosystems depending on the spatial scale and environment (e.g., water exchange rate and primary production). However, ecological impacts of suspended oyster farming at different scales have not been compared or comprehensively analyzed. To achieve environmentally sustainable shellfish aquaculture, it is essential to understand interactions between coastal marine environments and farming activities at multiple scales.
The goal of our study was to examine the interactions between suspended oyster farming and surrounding biogeochemical environments in Shizugawa Bay at two spatial scales, the farming area and bay scales, and further characterize the current status of the bay in terms of oyster production and environmental sustainability. First, we tested the effects of oyster farming on the biogeochemical properties of the surrounding biogeochemical environment, such as sediment and water chemistry, at a relatively small spatial scale, ca. 10-2 km2, by comparing the inside and outside of an oyster farming area. Second, we evaluated interactions between oyster farming and the macro-scale POM concentrations in the bay. Specifically, we estimated an approximate budget of particulate organic carbon (POC) based on measurements of POC fluxes, such as primary production and respiration of planktonic community, and POC exchange across bay boundaries. Based on these examinations, we discuss the environmental impacts of oyster aquaculture and sustainability of oyster production in the bay.
II. MATERIALS AND METHODS
Study site
This study was conducted in an inner part of Shizugawa Bay, located in the northeast of Japan (Fig. 1a). The studied area of bay has a surface area of 16 km2 and a mean depth of 20 m. The annual range of seawater temperatures in 2-m depth is approximately 5 to 21 °C.
Environmental measurement in the oyster farm
Sampling ̶ The oyster-farming site, OY2, has an average depth of ca. 22 m (Fig. 1b & 1c). In the farm, oyster clusters growing on scallop shells are tied to ropes at ca. 0.5-m intervals and suspended at a depth ranging from approximately 1 to 10 m. The influence of the suspended oysters on surrounding biogeochemical environments was examined by comparing the inside, edge, and outside of the oyster farm in the afternoon of August 4, 2014. In the oyster farm, 2-year-old Pacific oysters (C. gigas) were cultured, but no oysters were suspended at the edge points of the
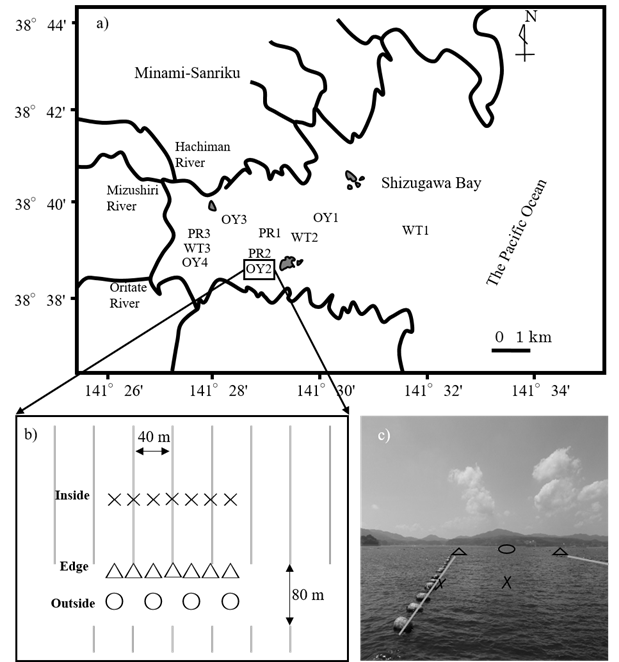
Fig. 1. a) Location of all sites studied in Shizugawa Bay. b) Sampling sites inside, edge, and outside of the studied longlines (showed by grey solid lines) in station OY2 are indicated by×, △ and ○ symbols respectively. c) Image of sampling sites in station OY2.
farm. The subsurface seawater (ca. 50 cm) for Chl.a measurements was collected at 9 of 18 locations at the inside, edge, and outside of the oyster farm using a bucket. To measure the organic carbon content of the sediment, the 5-mm subsurface sediment was also collected at the 18 locations using an Ekman-Birge bottom sampler. Furthermore, the concentrations of dissolved oxygen (DO) and Chl.a were measured at a 0.3 m above the bottom at the 18 locations using a CTD sensor (AAQ1183, JFE Advantech CO., LTD, Nishinomiya, Hyogo, Japan).
Chemical analyses ̶ For the analysis of Chl.a in subsurface seawater, 2 L of seawater from each sample was filtered through a glass fiber filter (GF/F, Whatman). Filtered samples were ground and extracted in approximately 10 mL of 90% acetone, and the concentration of Chl.a was spectrophotometrically determined [12] (UV-1800, SHIMADZU CO., LTD, Kyoto, Japan). To estimate the organic carbon content of sediment, the sediment samples were sifted through a 250-μm sieve, acidified with 10% HCl to remove carbonate, and then dried at 60°C. The carbon content of sediment samples was estimated using an elemental analyzer (FLASH 2000, Thermo Scientific, Waltham, MA, USA).
Statistical analyses ̶ To assess the differences in the chemical indicators between the inside, edge, and outside locations, one-way analyses of variance were performed. A p-value of <0.05 was considered significant.
Oyster growth experiment
To determine the oyster growth rate, Pacific oysters (C. gigas) were grown at four stations in the bay: Stns OY1, OY2, OY3, and OY4 (Fig. 1a). At each station, in each of three nylon net cages, approximately 50 juvenile oysters with an initial soft tissue biomass of 0.50 ± 0.38 g-wet ind-1 were introduced, and the cages were suspended at a depth of approximately 2 m using a rope. The cages and oyster shell surfaces were cleaned using a brush every 1 to 2 months to remove sessile organisms. At each station, ten individual oysters were randomly sampled from the cages one year after the experiment began. The shell length and soft tissue biomass of the oysters were measured. The juvenile oysters were collected by placing plastic plates in the bay from August to September of 2014, and the growth experiment was started at all stations in October 2014.
To calculate oyster biomass at carbon base, the conversion factors [dry weight]/[wet weight] and [carbon weight]/[dry weight] of 0.19 ± 0.02 (mean ± sd) and 0.51 ± 0.07, respectively, were used; these were determined in our preliminary analyses in August 2014.
POC budget estimation
To evaluate the effects of oyster aquaculture on POC dynamics and the carrying capacity for oyster production in Shizugawa Bay, a daily POC budget during the summer in calm weather conditions was estimated. The fluxes of POC-related processes were estimated under some assumptions, described below.
POC fluxes across boundaries of the bay ̶ To determine POC fluxes from adjacent watersheds via the rivers, monthly water sampling was conducted from June 2014 to June 2015 at the lowest freshwater reaches of the three rivers—the Hachiman, Mizushiri, and Oritate Rivers (Fig. 1a)—flowing into the inner part of the bay. The largest river flowing into the bay, Hachiman River, has a discharge rate of approximately 4 m3 s-1 at summer base flow [13]. For convenience, the total base flow discharges from all rivers flowing into the bay was assumed to be 10 m3. Since the POC concentration did not significantly differ between the three samples rivers, a pooled average of the monthly sampling data for the three rivers was used.
Likewise, for POC analysis, monthly water sampling was conducted from June 2014 to June 2015 at the three stations in the inner (WT3), middle (WT2), and outer (WT1) parts of the bay (Fig. 1a). Since the POC concentration did not differ significantly between the three stations, a pooled average of the monthly sampling data was used for the stations. We assumed that POC is exchanged only by the tidal exchange of seawater associated with a 1-m difference between high and low tides, and that the POC concentration at the boundary between the bay and the offshore area does not change between ebb and flood tides.
To determine the flux of POC deposition in the bay, three sediment traps were deployed inside and outside of the oyster farm, OY2, on August 5 and 6, 2014. The trapped materials were analyzed for POC.
The particulate materials were collected on glass fiber filters from the water and sediment trap samples by filtration, acidified and analyzed for POC by the elemental analyzer mentioned above.
Primary productivity and respiration by the plankton community ̶ The rates of primary production and respiration by plankton communities were determined using the light-dark bottle method on September 15, 2015 at three stations (PR1, PR2, and PR3) in the bay (Fig. 1a). The water depths of the three stations ranged from 10 to 22 m. The seawater was collected from three to four layers at each station using a Bandon water sampler, and 100 mL of seawater was sealed in two glass bottles, one of which was further enclosed in an opaque plastic bag. The DO concentration in the bottles was measured using a DO sensor (Microx 4, PreSens, Regensburg, Germany). Those bottles were tied to a rope, suspended at each sampling depth, and incubated in situ for 3 to 4 hours. The incubation was conducted in calm, sunny conditions, and the water temperature of the seawater ranged from 19.5 to 21.6 °C during the incubation period. The rates of primary production and respiration differed between depths, but generally did not differ between sampling stations. Thus, for the sake of convenience, to estimate the POC budget, we assumed rate homogeneity in the bay, and averaged the data from all stations and the depth layers to obtain representative rates in the bay.
Oyster feeding ̶ The feeding rates of oysters were determined in a mesocosm experiment in November 2014. Each of three 100-L mesocosms was supplied with 1.5 kg of wet-weight 16-month-old oysters, and they were tied to a fishing boat mooring in a harbor of the bay. The seawater of the harbor was pumped to a head tank placed on the boat and continuously added to each mesocosm at a residence time of 1 hour. The experiment was conducted for 22 hours and seawater samples were collected at the beginning and end of the experiment from the head tank and all mesocosms. Based on the difference in POC concentration between the head tank and mesocosms, the filtration rate of POC by the oysters was estimated. The seawater temperature during the mesocosm experiment was 12.8 °C.
To estimate the total oyster feeding in the entire bay, quantitative information regarding the oyster culture conditions was obtained (e.g., the numbers of aquaculture facilities, suspension ropes, and clusters, and oyster density within clusters) based on our preliminary sampling in August 2014 and interviews of the local fishery committee.
III. RESULTS
Biogeochemical environment around the oyster farm
The concentration of Chl.a in the seawater was higher in the bottom layer than the surface lay for all horizontal locations: inside, edge, and outside of the oyster farm (Fig. 2a & 2b). Furthermore, there were no significant differences in Chl.a concentrations among the horizontal locations in the surface or bottom water (p = 0.86 & 0.21, respectively).
The concentration of DO in the bottom-layer seawater differed significantly among the inside, edge, and outside of oyster farm (p = 0.008). Specifically, the DO concentration was lower at the edge sampling points than the other points (Fig. 2c). Furthermore, the DO concentration was positively related to Chl.a concentration in the bottom-layer seawater (R2 = 0.46, p = 0.002). The organic carbon content was higher, on average, at the edge sampling locations of the oyster farm than the other locations (Fig. 2 d), but it did not exhibit a significant relationship with the DO concentration (R2 = 0.009, p = 0.72).
Oyster growth rate
The oyster growth rate was approximately 5.0 ± 1.0 g-DW ind-1 yr-1 and 2.5 ± 0.5 g-C ind-1 yr-1. The number of oyster individuals in each cluster was estimated at 23.1 ± 13.3. Thus, the total number of aquacultured oysters and the production rate of oysters in the entire bay were ca. 26.3 million individuals and ca. 65.8 ton-C yr-1, respectively.
Marco-scale POC budget of the bay
The rates of primary production and respiration in the seawater were generally 10 times
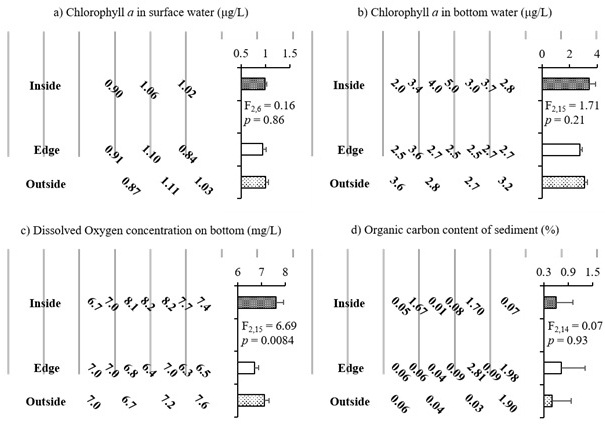
Fig. 2. Chemical environment surrounding the suspended oyster farming longlines in 2014. a) Chl. a concentration in surface (μg/L), b) Chl. a concentration in bottom water (μg/L), c) DO concentration on bottom (mg/L), d) Organic carbon content of sediment (%).
greater than the other fluxes, indicating that the two processes have the greatest effect on POC dynamics in the bay (Fig. 3). The flux due to filter feeding by oysters was one to two orders of magnitude less than those of POC input from offshore systems and net primary production in the seawater.
IV. DISCUSSION
Our POC budget estimates at the macro, bay scale demonstrated two important points. First, the primary production and respiration in the seawater probably had the greatest effects on the POC budget in the bay, overwhelming other processes in organic matter dynamics. Second, the feeding rate of entire aquacultured oysters in the bay was much lower than the fluxes of net primary production and input of offshore POC. The fluxes of POC dynamics were all temporally and spatially variable, but the oyster feeding rate was two orders of magnitude less than the net primary production rate. In addition, the flux of offshore POC input was probably a conservative estimate, since we only accounted for the tidal input of POC for convenience, and neglected advective POC inputs across the bay-offshore boundary due to coastal currents. Thus, based on our POC budget estimates, POC is probably sufficient for oyster production, at least during warm and relatively biologically productive seasons in Shizugawa Bay. Furthermore, current oyster farming is less likely to affect the entire POC budget at the bay scale. However, our estimates did not consider temporal variability in POC fluxes. To conclusively understand the interactions among POC dynamics and oyster aquaculture in the bay, further investigations of temporal variation in POC fluxes and annual base estimates of the POC budget are needed.
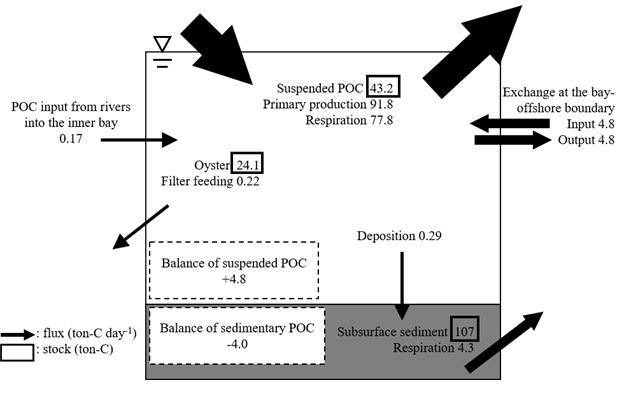
Fig. 3. Macro-scale POC budget in Shizugawa Bay.
At a local scale, we detected some significant effects of oyster farming on local biogeochemical parameters. The Chl.a concentration of seawater was low in the surface layer than the bottom layer. This lower concentration of Chl.a may be due to filter feeding by oysters, since oysters are suspended at depths of <10 m. In addition, the deposition of algal materials originating from phytoplankton and periphyton at aquaculture facilities may contribute to the relatively higher Chl.a concentration in the bottom layer. Unexpectedly, we also found relatively lower Chl.a and DO concentrations in the bottom layer at the edge sampling points of the oyster farm than at the other sampling points. This may be due to a lack of periphyton deposition from oyster shells and farming facilities. Meanwhile, since the DO concentration near the bottom was greater than 6.0 mg/L and was positively related to the Chl.a concentration, the photosynthetic activity of algal materials deposited at the bottom may have minimized the depletion of DO near the bottom. It should be noted that our field investigation was conducted during the daytime. During the night, the respiration of algal materials may increase DO depletion, and possibly result in a negative relationship between Chl.a and DO concentrations in the bottom layer. Our results suggest that the deposition of exfoliated materials from oyster farm facilities significantly affects local biogeochemical environments surrounding oyster farms. The cumulative effects of oyster farming on the surrounding environment need to be carefully monitored in the bay. To mechanistically understand and properly assess the environmental impacts of oyster farming, additional studies with sampling at a higher spatial resolution and the determination of fluxes (e.g., periphyton exfoliation and the deposition and dispersion of organic particles) are needed.
Whereas there was likely a negligible effect of oyster aquaculture on POM dynamics at the macro, bay scale, we found significant influences of oyster farming on biogeochemical environments at the local, oyster farm scale. Similarly, refs. [7] and [14] examined the effects of mussel feeding on the Chl. a concentration of seawater at two different scales by a field survey and mesocosm experiment, and reported contrasting results; Chl. a decreased due to filter feeding in the mesocosm, and increased via increased nutrient regeneration in the field (Table 1). These results indicate that the impacts of bivalve aquaculture depend on the spatial scale. The current oyster
Table 1. Comparison among studies of the impacts of bivalves on phytoplankton biomass at various scales, including microcosms, and mesocosms, and field studies
Sp: Spring, Su: Summer, Au: Autumn, Wi: Winter, WW: Wet Weight, DW: Dry Weight, DIN: Dissolved Inorganic Nitrogen; DIP: Dissolved Inorganic Phosphorus; PP: Phytoplankton Production, NA: Not Available
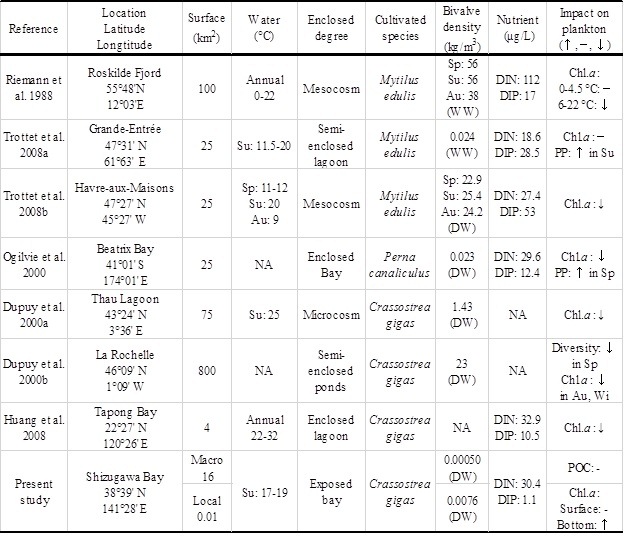
density in the bay, which was approximately one order of magnitude lower than the density at the local, oyster farm scale, may not be high enough to impact the entire bay.
Based on a review of previous studies, shellfish aquaculture is likely to substantially affect environmental parameters (e.g., the Chl. a concentration of seawater) at both small and large spatial scales. In particular, cultivated bivalves tend to be related to significant decreases in POM in seawater based on previous studies, and this general result is likely explained by filter feeding. However, phytoplankton production could also increase, while mussels seem to supply nutrients to local environment with low nutrient level. These previous studies generally examined relatively enclosed bays or small experimental systems. Compared with the previous study sites in examinations of the impacts of bivalve aquaculture, Shizugawa Bay is more exposed to the offshore ocean system. In exposed bays, materials in the seawater such as plankton and nutrients tend to be spatially homogenized by wind and coastal currents [15], and are exchanged at high rates across the boundary between the bay and offshore system. This may promote the maintenance of a consistent nutrient supply and primary production in the bay, although the concentrations of nutrients, particularly inorganic phosphorous, as well as Chl.a were relatively lower (Fig. 4). These also may explain the reduced impact of oyster farming at a macroscale on the POC budget in the bay.
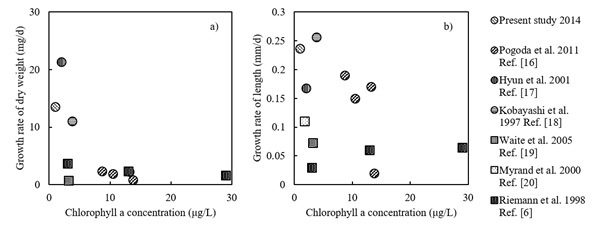
Fig. 4. Correlation between Chl. a concentration and the growth rate of bivalves (○ symbols for oysters: Crassostrea gigas and □ symbols for mussels: Mytilus edulis) a) dry weight b) length.
Compared with previous studies, in Shizugawa Bay, the oyster growth rate is relatively higher, despite the relatively lower Chl. a concentration. As mentioned above, the relatively high exposure of the bay may also contribute to consistent nutrient input and enhanced primary production in the bay. In addition, nutrient cycling is an important process in the maintenance of biological production. Oysters have been reported to increase biodeposition, nutrient excretion, and phytoplankton productivity inside farming areas [21]. To identify processes supporting the increased production of oysters in the oligotrophic bay, further studies should examine the precise origins of nutrients that sustain primary production in the bay.
V. ACKNOWLEDGEMENTS
We acknowledge financial support from The Environment Research and Technology Development Fund #S-13 and The Japanese Institute of Fisheries Infrastructure and Communities. We also appreciate the assistance with field and laboratory work by K. Goto, T. Kudo, N. Chiba, A. Kato, C. Maruo, H. Kanzaki, H. Itakura, T. Ikeda, S. Ohira, Y. Taguchi, Y. Hata and N. Hayashi.
1. S.E. Shumway, Shellfish Aquaculture and the Environment, WILEY-BLACKWELL, 2011.
2. M. Nomura, N. Chiba, K.Q. Xu, and R. Sudo, “The Formation of Anoxic Water Mass in Shizugawa Bay,” Coastal Oceanography Research, 2006, vol. 33, pp. 203-210, [in Japanese].
3. J.M. Rose, S.B. Bricker, M.A. Tedesco, and G.H. Wikfors, “A Role for Shellfish Aquaculture in Coastal Nitrogen Management,” Environ. Sci. & Technol., 2014, vol. 48, pp. 2519-2525.
4. D. Brigolin, G.D. Maschio, F. Rampazzo, M. Giani, and R. Pastres, “An individual-based population dynamic model for estimation biomass yield and nutrient fluxes through an off-shore mussel (Mytilus galloprovinialis) farm,” Estuar. Coast Shelf S., 2009, vol. 82, pp. 365-376.
5. C. Dupuy et al., “Feeding rate of the oyster Crassostrea gigas in a natural planktonic community of the Mediterranean Thau Lagoon,” Mar. Ecol. Prog. Ser., 2000, vol. 205, pp. 171-184.
6. B. Riemann, T.G. Nielsen, S.J. Horsted, K.P. Bjørnsen, and J. Pock-Steen. “Regulation of phytoplankton biomass in estuarine enclosures,” Mar. Ecol. Prog. Ser., 1988, vol. 48, pp. 205-215.
7. A. Trottet, S. Roy, E. Tamigneaux, C. Lovejoy, and R. Tremblay, “Impact of suspended mussels (Mytilus edulis L.) on plankton communities in a Magdalen Islands lagoon (Que´bec, Canada): A mesocosm approach,” J. exp. Mar. Biol. Ecol., 2008, vol. 365, pp. 103-115.
8. S.C. Ogilvie, A.H. Ross, and D.R. Schiel, “Phytoplankton biomass associated with mussel farm in Beatrix bay, New Zealand,” Aquaculture, 2000, vol. 181, pp. 71-80.
9. C. Dupuy, A. Pastoureaud, M. Ryckaert, P.G. Sauriau, and H. Montanié, “Impact of the oyster Crassostrea gigas on microbial community in Atlantic coastal ponds near La Rochelle,” Aquat. Microbial Ecol., 2000, vol. 22, pp. 227-242.
10. C.H. Huang, H.J. Lin, T.C. Huang, H.M. Su, and J.J. Hung, “Responses of phytoplankton and periphyton to system-scale removal of oyster-culture racks from a eutrophic tropical lagoon,” Mar. Ecol. Prog. Ser., 2008, vol. 58, pp. 1-12.
11. J.M. Pietros, and M.A. Rice, “The impacts of aquacultured oysters, Crassostrea virginica (Gmelin, 1791) on water column nitrogen and sedimentation: results of a mesocosm study,” Aquaculture, 2003, vol. 220, pp. 407-422.
12. K. Kawakita, Clean water test method, Japan Water Works Association, 2001. pp. 563-566.
13. K. Matsuyama, H. Tanaka, and N. Shuto, “Field observation of water chemistry during flood events,” Research symposium of JSCE-Tohoku, 1993, [in Japanese].
14. A. Trottet, S. Roy, E. Tamigneaux, C. Lovejoy, and R. Tremblay, “Influence of suspended mussel farming on planktonic communities in Grande-Entree Lagoon, Magdalen Islands (Quebec, Canada),” Aquaculture, 2008, vol. 276, pp. 91-102.
15. T.C. Prins, A.C. Smaal, and R.F. Dame, “A review of the feedback between bivalve grazing and ecosystem process,” Aquat. Ecol., 1998, vol. 31, pp. 349-359.
16. B. Pogoda, B.H. Buck, and W. Hagen, “Growth performance and condition of oysters (Crassostrea gigas and Ostrea edulis) farmed in an offshore environment (North Sea, Germany),” Aquaculture, 2011, vol. 319, pp. 484-492.
17. K.H. Hyun et al., “The effect of food composition on Pacific oyster Crassostrea gigas (Thunberg) growth in Korea: a modeling study,” Aquaculture, 2001, vol. 199, pp. 41-62.
18. M. Kobayashi, E.E. Hofmann, E.N. Powell, J.M. Klinck, and K. Kusaka, “A population dynamics model for the Japanese oyster, Crassostrea gigas,” Aquaculture, 1997, vol. 149, pp. 285-321.
19. L. Waite, J. Grant, and J. Davidson, “Bay-scale spatial growth variation of mussels Mytilus edulis in suspended culture, Prince Edward Island, Canada,” Mar. Ecol. Prog. Ser., 2005, vol. 297, pp. 157-167.
20. B. Myrand, H. Guderley, and J.H. Himmelan, “Reproduction and summer mortality of blue mussels Mytilus edulis in the Magdalen Islands, southern Gulf of St. Lawrence.,” Mar. Ecol. Prog. Ser., 2000, vol. 197, pp. 193-207.
21. A. Smaal, M.v. Stralen, and E. Schuiling, “The interaction between shellfish culture and ecosystem processes.” Can. J. Fish. Aquat. Sci., 2001, vol. 58, pp. 991-1002.







